Methane is emitted by a myriad of naturally occurring and anthropogenic biogenic sources in nature including shellfish, plankton, beaver ponds, rice paddies, wetlands, termites, arthropods, ruminants, dams, trees, or just about any place methanogenic archaea reside in anoxic environments (including soil and digestive tracts) or by non-methanogenic cells occurring in oxic environments (Ernst et al 2021). There’s also methane emitted by thermogenic (fossil fuels) and pyrogenic (fire) sources. So…no, methane isn’t only emitted by belching cows as some, it seems for financial or quasi religious reasons, would like you to believe (Lakhani et al. 2017).
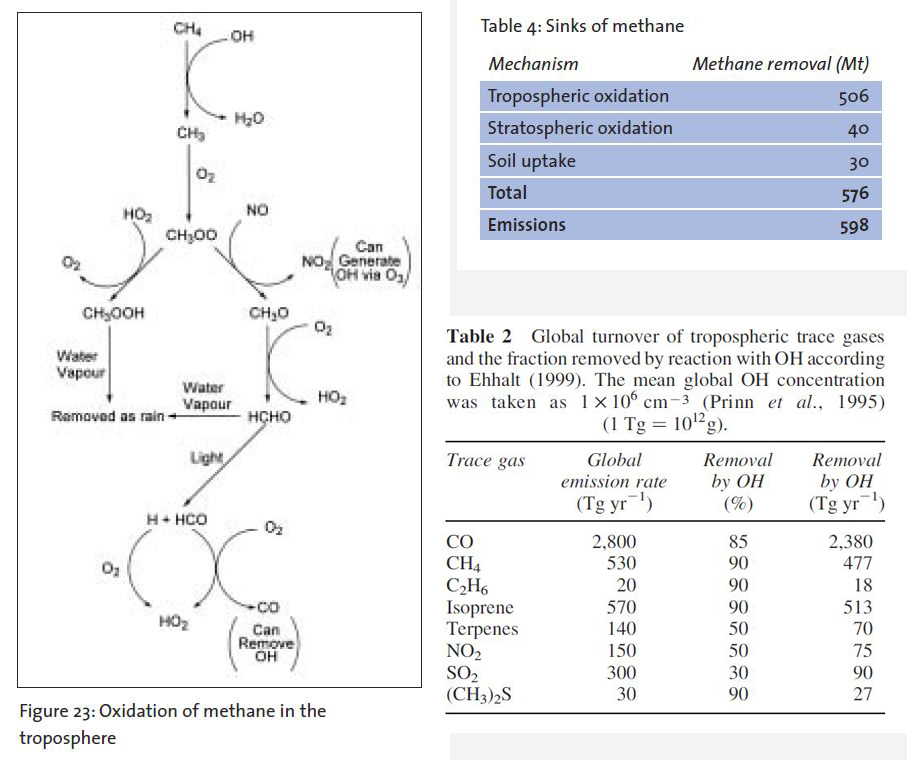
As I’ve noted in previous blog entries (e.g. WTF happens to all that methane?), all of this methane is relatively quickly oxidized back to CO2 and water by methanotrophs in the soil and hydroxyl radicals [OH] in the troposphere. The soil sink is small relative to the tropospheric sink (Prinn 2014). The amount and persistence of methane in the much larger tropospheric sink is largely contingent on OH availability (Rigby et al 2017).
OH availability is dependent on a number of variables including:
1. Ability of ecosystem to make OH as noted in my blog, Context matters: Green zone hydroxyl radical formation and methane oxidation. So less plants (from desertification) equals less water vapor and less BVOC’s which are key ingredients needed for OH formation (Monk et al. 2015) .
2. Greater amount of other methane using OH. So more methane and the same amount of OH means there’s less OH to oxidize methane. That’s been a big problem with industrialization plus the use of conventional and fracked natural gas…since all of the thermogenic sources of methane (cold bed gas, coal, fugitive natural gas emissions, etc) have pumped old trapped sources of methane into the atmosphere. According to one recent study (Holmes 2018) methane persistence has gone from 5.9 to 9.2 years up to 7.3 to 13.7 years due to more thermogenic methane emissions:
3. Higher amounts of other trace gases using OH…since OH scrubs a lot of other trace gases from the atmosphere…not just CH4 (Prinn 2014). This includes CO….which collides with OH and forms CO2. One big source of CO is fire…so more forest fires equals more CO (and to a lesser degree more pyrogenic CH4) …and thus less OH for methane oxidation (Rowlinson et al. 2019).
4. All of the above. So, less OH formation due to desertification and less availability due to competitions from more thermogenic & pyrogenic methane emissions as well as more other trace gas emissions. Thus weakened OH sinks and greater CH4 emissions, a double whammy.
The thing too is that oxidized CH4 breaks down to CO2 and H2O……So if there’s more desertification, bare fallows, weakened photosynthesis from weakened plants, etc… than there’s less photosynthesis and less root exudations. And thus more persistence of the CO2 from CH4 oxidation. Less carbon in the soil also screws up the water cycle, so less water penetration and retention…thus even less vegetation and photosynthesis. So again weakened sinks concurrently occurring with greater indirect (via CH4) and direct CO2 emissions particularly from fossil fuels. Less plants also means less water vapor and BVOC (per #1 above) so less OH available for secondary oxidation to form ice nucleating particles…and thus less consolidation of water vapor in clouds and less predictable rain fall as I discussed in my prior blog, Interdependent cycles and rainfall.
So unless we reduce emissions that exceed the biogenic carbon cycle, while we simultaneously restore sinks, we won’t rebalance the carbon cycle. Rebalancing the carbon cycle (i.e. emissions are offset by sinks) entails the need to increase and maximize photosynthesis by regenerating our rangelands, agricultural systems, mangroves, wetlands, and other ecosystems as my friend Paul Hawken ably illustrates in his wonderful new book, Regeneration: Ending the climate crisis in one generation. Otherwise, our planet Earth will slowly becomes more and more like Mars.
References:
Lakhani, N et al. 2017. Methanogenesis: Are ruminants only responsible: A review.
Ernst, L. et al 2021. Methane formation driven by reactive oxygen species across all living organisms
Prinn, R.G. 2014 Ozone, Hydroxyl Radical, and Oxidative Capacity
Rigby et al 2017. Role of atmospheric oxidation in recent methane growth.
Monk, P.S. et al. 2015. Tropospheric ozone and its precursors from the urban to the global scale from air quality to short-lived climate forcer
Holmes, C. D. 2018. Methane Feedback on Atmospheric Chemistry: Methods, Models, and Mechanisms
Rowlinson, M et al 2019 Impact of El Niño Southern Oscillation on the interannual variability of methane and tropospheric ozone.
3 thoughts on “Methane persistence and hydroxyl radical availability”