Okay this one is a bit nerdy, so I apologize in advance. When I’m not so lazy, I’ll track down all the scientific references, but for now I just want to summarize all of these compiled processes in what follows below.
Methane emitted from a number of various sources doesn’t just rise into atmosphere unabated. That methane is actually always in the process of being broken down via a process called oxidation. In soils, what oxidizes microbial methane (made by single celled prokaryotes called methanogenic archaea) before it can get into the atmosphere are bacteria called methanotrophs. Once in the atmosphere, these bacteria still oxidize a small amount of this methane, but not a whole lot: Maybe around five percent.
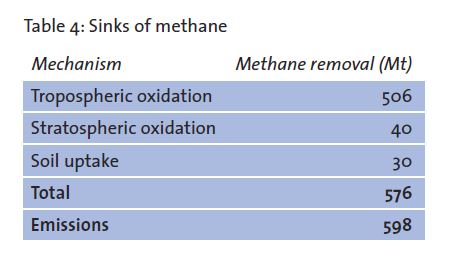
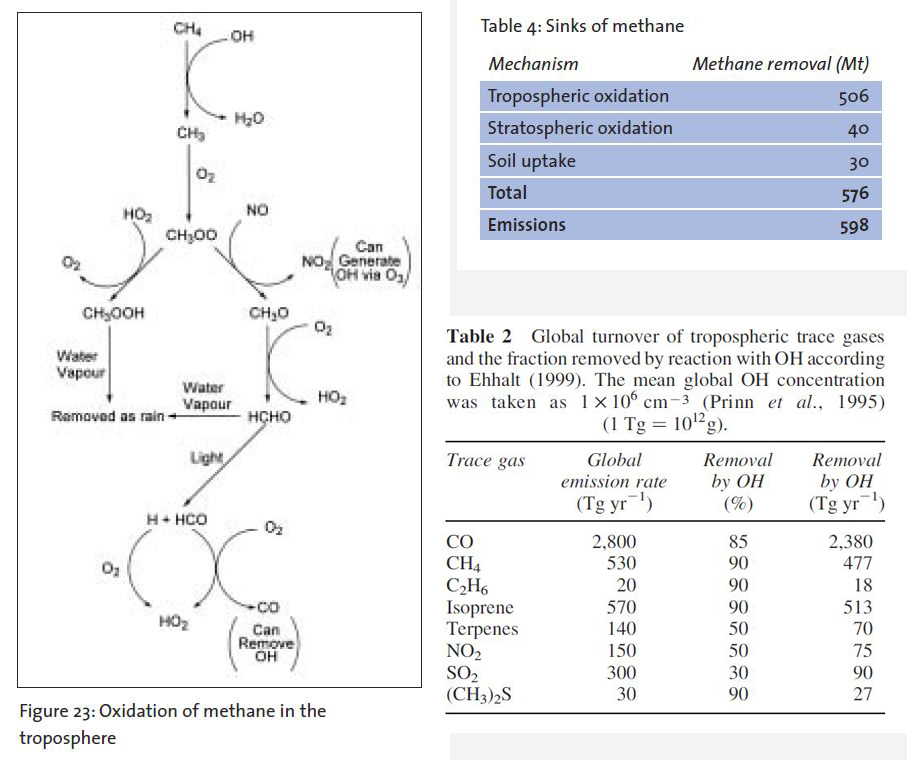
However, once methane is in the atmosphere this is where all the real oxidation happens. This is where methane [CH4] collides with hydroxyl radicals and is ultimately reduced back to water H2O and cyclical CO2. Note the word cyclical since most atmospheric CO2 cycles via photosynthesis to glucose and oxygen O2 as a by product whether it goes through a human, a head of cattle or a kangaroo.
These monoterpines then react with nitric oxide released from soil to form longer nitrates …then those nitrates are zapped with light via photolysis to form ozone O3 which then is zapped as noted above and combined with water vapor to form the hydroxyl radicals. Those radicals are negatively charged free radicals that steal one of the hydrogens off of a CH4 molecule which starts a long process of breaking down CH4 to essentially H2O and cyclical CO2. as per the attached diagram (Figure 23 above).
Capiche?
Anyway, as a interesting side note, the BVOC’s also interact with the hydroxyl radicals and these oxidized BVOC’s then become the nuclei required for rain cloud formation, so trees and plants actually help produce rain.
Very interesting article! I don’t mind the nerdiness one bit 😂 You mention methanotrophs in your article. I posted a piece yesterday about how we may be able to mimic the conversion process they perform (methane to methanol) and create fuel from atmospheric greenhouse gases. Here’s the link if you’re interested https://adambolandblog.com/2019/06/12/gas-in-the-tank/
Thanks again for the great piece! 😁
LikeLiked by 1 person
A great summary you have provided there. Very useful. Thanks.
LikeLike