Too often nowadays it seems for financial or quasi religious reasons, conflicted scientists purposely filter information to confirm and give credence to their biases. This is especially the case with advocates masquerading as scientists.
Take for example, Carter & Urbancic’s “Seeing Stars” white paper a friend recently sent me to read. This paper is an excellent example of filtering to further a preferred narrative. Filtering is a common cognitive distortion that many people exhibit (and plant based diets seem to exacerbate).
Take for example these two sentences from this paper, where the authors are arguing for the need to drastically reduce methane emissions particularly from domesticated ruminants:
“…Methane’s impacts go beyond warming. Methane concentration also contributes to ground-level ozone formation (otherwise known as smog) which causes roughly one million premature deaths each year around the world…”
Yes, the hydrocarbon methane CH4 is a precursor for ozone O3 formation, and surface level (tropospheric ozone) is a pollutant. For oxidized CH4 to form ozone, its intermediate products (eg. hydrogen peroxyl radical HO2) needs to react with nitrogen oxides (NOx) to provide an oxygen radical to bind with oxygen O2 to form O3 as follows:
CH4 + O2—-> HO2 + CH3R
HO2 + NO—> OH + NO2
NO2 + hv–> NO + O
O + O2 —> O3
(hv in the equation above is photon/light at a certain wavelength).
However, that isn’t the entire story.
Any hydrocarbon is a precursor for O3 formation including volatile organic compounds (like benzene C6H6, toulene C6H5CH3, formaldhyde CH2O, etc) and biological volatile organic compounds (like isoprene C5H8, methanol CH4O, etc). All of these compounds also react with NOx to free up oxygen O radicals to bind with O2 to form O3. BVOC’s are emitted by plants. NOx can come from a variety of places including soils, fertilizers, combustion, biomass burning, and lightning. NOx can be nitric oxide NO, nitrites NO2 or nitrates NO3.
Ozone can be formed a number of naturally occurring and/or man made anthropogenic ways from VOC’s or BVOC’s or various sources (biogenic, pyrogenic, thermogenic) of CH4’s intermediates reacting with NOx from tail pipes, soil or other natural or man made sources.
Here’s an example of the combustion of CH4 reacting with atmospheric oxygen O2 and nitrogen N2
CH4 + 2O2 + N2–> Co2 + H2 + NO2
NO2 + hv–> NO + O (hv = energy from photons)
O + O2 —> O3
So, I guess, per “Seeing Stars” authors’ logic to prevent tropospheric ozone from forming from hydrocarbons, we should also chop down all trees and plants since these emit BVOC’s. Moreover, per this logic, we should also pave over all soil so that soil won’t emit any of nitrogen oxides NOx needed for ozone formation.
Oh, and I forgot to mention that ozone O3 can also be formed from carbon monoxide CO as follows:
CO + 2O2 + hv –> CO2 + O3
But here’s the real kicker: The primary pathway for formation of hydroxyl radicals OH that are needed to oxidize CH4 and other trace gases in the atmosphere is via the photolysis of tropospheric ozone. So without O3 you have a lot less OH to break down CH4, and thus CH4 is a lot more persistent.
O3 + hv —> O + O2
O + H2O —> 2OH
Or, in other words, surface level ozone isn’t always a “bad” thing. Again for emphasis, without O3, there’s a lot less OH. And as I detail below, less OH means there’s more methane. Though the real problem with ozone is having too much in urban areas forming from the NOx from combustion emissions….not from biogenic precursors.
A lot of atmospheric chemistry reactions go both ways or are circular depending upon different variables like photon wave length, temperature, etc.
Additionally, as the Shiny Stars’ authors correctly noted CH4’s half life is “on average” [9 to] 12 years.
CH4’s persistence depends on OH availability. OH lasts less than a second in the atmosphere. So where OH is more available, CH4 and other trace gases can be broken down quickly. But near the tropopause and in the stratosphere where OH is scarce, CH4 may take as long as 140 years to break down (Stevenson, D. S. et al. 2020). So, the more OH availability, the lower the CH4’s lifespan. The shorter the lifespan, the less atmospheric CH4 accumulation.
The paper (Holmes 2018) the Seeing Stars authors cited about pre-industrial levels actually had CH4’s half life at 5.9 to 9.2 years. The current average half life is 7.3 to 13.7 years. So that’s more than the 25% difference that Carter & Urbancic claimed . According to Holmes, due to more CH4 from thermogenic sources of CH4 from industrialization and the resulting decrease in OH availability, there’s been more like a 55% increase in lifetime of CH4 in the atmosphere. Note too the longer CH4’s lifespan, the more it compounds and the higher the atmospheric CH4 concentrations.
So, more methane emissions from all sources of methane reduce OH availability. And more carbon monoxide CO emissions do as well, especially from combustion of fossil fuels and from forest fires. CO binds with OH to form CO2 and HO2 as follows:
CO + OH + O2 –> CO2 + HO2
BVOC’s also react with OH to form secondary organic aerosols [SOA’s]. So BVOC’s also make OH less available….but as noted above BVOC’s are also precursors for O3 formation. So BVOC’s are also needed for OH formation. (SOA’s act as the nuclei in clouds to consolidate water vapor into rain as I discussed in this old blog, Interdependent cycles and rainfall).
Thus, as noted above, atmospheric chemistry isn’t so simple. It’s quite convoluted. “Seeing Stars” authors cherry picked and filtered their limited understanding of this chemistry to fit their preferred scientific narrative and, as I explain further below, that’s to blame rises in atmospheric methane primarily on livestock emissions due to increase of domestic herd sizes. So Carter & Urbancic specifically maligns biogenic enteric methane from domesticated livestock and firmly believe climate change issues could be resolved if everyone just went plant based and vegan.
However, when differentiating between different sources of methane (thermogenic, biogenic, and pyrogenic) context matters quite a bit. Thus two questions need to be asked. First, does the source of methane have an ecosystem context? And second, does that ecosystem context also emit the precursors needed for OH formation, and thus methane oxidation? .
For example, biogenic methane emitted from a wetlands is in an ecosystem that also emits water vapor and, as shown above, water vapor is needed for OH formation.
As another example, enteric methane emitted in a grassland ecosystem will be emitted along with BVOC’s and water vapor emissions from the stomata of plants as well as with nitrogen oxides emissions from the soil. As shown above, BVOC’s and NOx are precursors for the O3 needed to free up an oxygen radical to react with water vapor to form OH. The OH formed here will quickly oxidize the enteric CH4.
I discussed this grassland ecosystem example in this old blog: Context matters: Green zone hydroxyl radical formation and methane oxidation.
This grassland ecosystem context is very different than cattle in a feedlot with bare ground where cattle emit enteric CH4, but that bare ground context doesn’t emit the precursors needed for OH formation.
This grassland context is also very different than a poorly managed fire ecosystem or deforested rain forest system where forest fires release pyrogenic CH4 along with 20 times more CO. Rather than create the precursors needed for OH formation, that released pyrogenic CH4 and CO uses up OH and thus reduces methane oxidation.
Furthermore, fugitive thermogenic CH4 from fracked and natural gas gets released into the atmosphere without any precursors for OH formation. Thus these emissions also do nothing to enhance the tropospheric methane sink needed for hydroxyl oxidation.
So, even though all forms of CH4 retain the same amount of latent heat, these different types and sources of CH4 emissions impact the overall global methane budget differently. Again this has to do with the contexts like those I described, and gave examples of, above.
Now it was interesting that Seeing Stars’ authors provided the 2017 Global Methane Budget with only the top down numbers. Top down numbers are derived via regional carbon isotope monitoring. It’s more accurate than bottom up analysis, but top down analysis still has some issues including incomplete coverage of the globe and overlapping of carbon signatures which leads to mis-attribution of CH4 sources. So these numbers aren’t as conclusive as they’re sometimes presented. They’re gross estimates. Plus more recent studies are showing a lot more emissions from aquatic environments (up to 40 to 53% of all methane emissions as noted in Rosentreter et al 2021). Satellite data is also showing a lot more thermogenic emissions (super sites) especially from natural gas extraction and land fills (GHGSAT 2022).
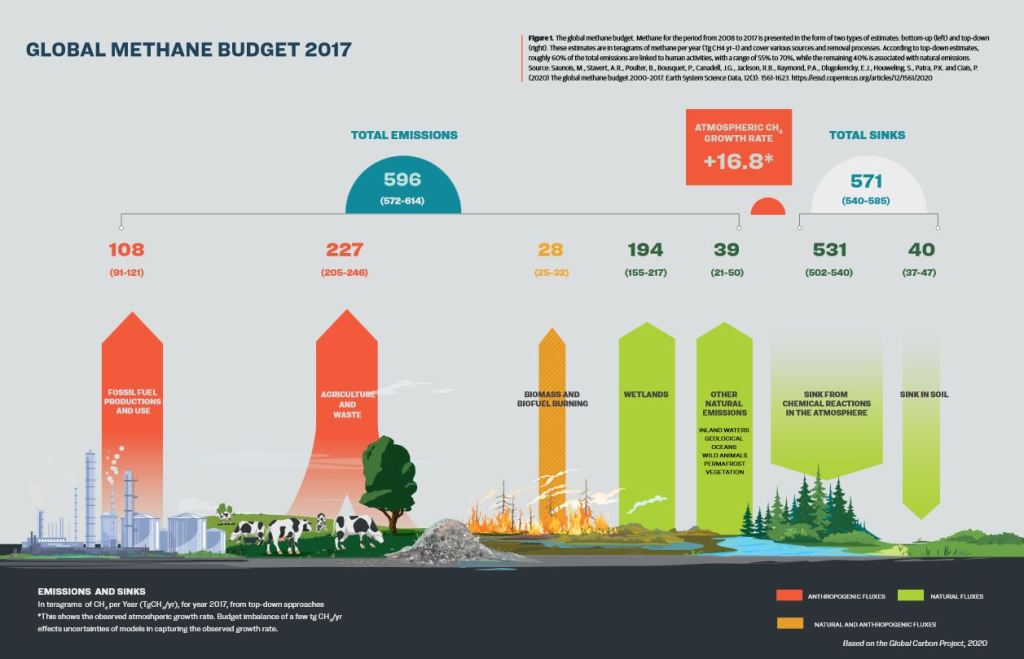
However, taking these numbers presented at face value, the difference between emissions and sinks is 596-571 or 25. That (compounding) excess is what leads to the increase of atmospheric CH4 levels. Thus if you reduced thermogenic emissions 25%, you’d more or less balance out CH4 emissions and sinks. Enhancing soil and atmospheric sinks slightly then helps create a deficit that leads to the reduction of atmospheric CH4.
Thus any emphasis on reduction of CH4 should be on those forms of CH4 that don’t also include OH precursor emissions. This would start with super sites like those being identified from satellite tracking. Thus when it comes to reducing methane emissions, the priority should be reducing fires, fossil fuel use and especially fugitive natural gas emissions, landfills, and intensive concentrated forms of industrial animal Ag.
However, if you instead take on the mindset of Seeing Stars authors, and the goal is to reduce all diffuse forms of CH4, then you might as well drain wetlands, peat bogs, water reservoirs, small ponds and extirpate beavers, Beaver ponds also emit CH4 (Whitefield et al. 2015). Therefore, further following the logic of the authors of this Seeing Stars paper, the best way to save planet Earth from climate change is to dehydrate it and turn it into Mars.
Now to further their anti-livestock narrative, Carter & Urbancic cited a paper by Dangal et al 2017 . This paper, of course, claimed that all CH4 increases since 1890 have been due to the increased herd sizes of domesticated livestock. So, of course, Seeing Stars authors completely forgot or never recognized all the other CO, CH4 emissions from coal, natural gas, fracked gas, etc that also decreased availability of OH and increased overall greater CH4 persistence despite their reference to Holmes 2018.
Another interesting couple things about Dangal et al 2017 was that this paper used bottom-up accounting methods and GWP100 for CO2 equivalents… both of which over account for enteric methane.
The problem with bottom-up accounting is that what’s easy to measured gets the most blame. So the enteric methane as determined by mask, chamber or chamber is a lot easier to measure and extrapolate than the methane emitted by saprophytic fungi in soils, kangaroo farts, blooming and decaying algae in waterways, phytoplankton, cock roaches, and a myriad of other things that emit methane. Plus bottom-up accounting hitherto hasn’t accounted for the copious amounts of methane emitted from the previously mentioned super sites detected by satellites in the past eight or so years.
And since GWP100 (or GWP20) doesn’t account for hydroxyl oxidation, it too over counts the impact of methane significantly despite all of Shining Star authors’ protestations and prolix language to the contrary. GWP* isn’t perfect, but it’s a lot more real world than GWP100.
One more interesting thing about Dangal et al 2017 is that this paper didn’t recognize that in many places, especially North America, domesticated ruminants largely replaced wild ones. So the net increase of CH4 from livestock, even without recognizing their flawed estimate accounting methods, wasn’t as large as Dangal et al 2017 or Shiny Stars’ authors contend. It probably was a wash in many places where wild ruminants roamed in large numbers (Manzano et al 2023).
Back in 2018, in my Ruminations- Methane math and context blog, I noted that atmospheric rises in CH4 over the past few hundred years more closely correlate with coal, gas and fracked gas use as follows (there were also time frames of negative correlation with cattle herd sizes):
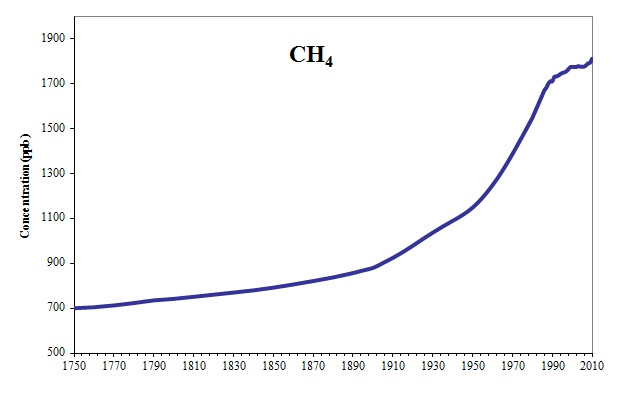
“…Looking at a graph of rising methane levels over the past 260 years (see graph above), most methane levels started to accumulate significantly during the industrial age and then rise rapidly from the 1950’s to the 1980’s before leveling off for a brief time between 1998 and 2007 (see graph below). CH4 levels started rising again in the past ten years. Prior to the use of coal, there were plenty of wild ruminants in North America as well as considerably much larger areas of wetlands, so there were plenty of natural occurring sources methane emissions. Though it’s hard to get exact numbers, estimates of 30 to 70 million bison, 30 million elk, 20 million pronghorn, etc exceed current domestic populations of ruminant in the United States (Hristov, A 2011). Globally wetlands have been significantly reduced. In California alone, 95% of wetland areas have been drained (Drexler et al. 2009). These naturally occurring sources of CH4 haven’t changed much. If anything emissions from wetlands and peat bogs have gone down….”
In summary, scientists, sophists, and advocates masquerading as scientists can make very compelling well referenced arguments by filtering content to further their preferred scientific or quasi-religious narratives. Carter & Urbancic’s Shiny Stars white paper is an example of such a well referenced paper. Shiny Stars selected and cherry picked from its references to fit the authors preferred world view where everyone eats plant based diets, and livestock are no longer raised for food.
But atmospheric chemistry, soil science, and climate science aren’t always so easily reduced to fit such preferred or agenda driven narratives. The natural world is infinitely complex with a lot of variables that don’t lend themselves to reductive equations and models. So, when we break down the natural world into more discernible parts, we have to again have a framework to better understand how all those different parts interact and create a whole. But sadly too often such holistic thinking is lacking in science especially profit or agenda driven science.
